Design evolution improves usability and patient care
It is important to monitor the hydration of critically-ill patients around the clock- which includes urine output. To address this need, the UnoMeter™ Safeti™ allows for hourly urine monitoring. When the time came to update this well-regarded product, Unomedical (part of ConvaTec) engaged PDD.
Our research in hospitals across Europe identifies opportunities for improved ease of use and infection control, without adding to product cost. By mapping the Safeti™ journey from purchasing, delivery and storage through to usage and disposal, a clear message emerged: while infection control remains paramount for healthcare professionals, they cannot pay a premium for it.
This insight ensured that PDD retained the best features of the original within the new UnoMeter™ Safeti™ Plus, while adding several incremental and cost-effective improvements to help usability, patient safety and appeal.
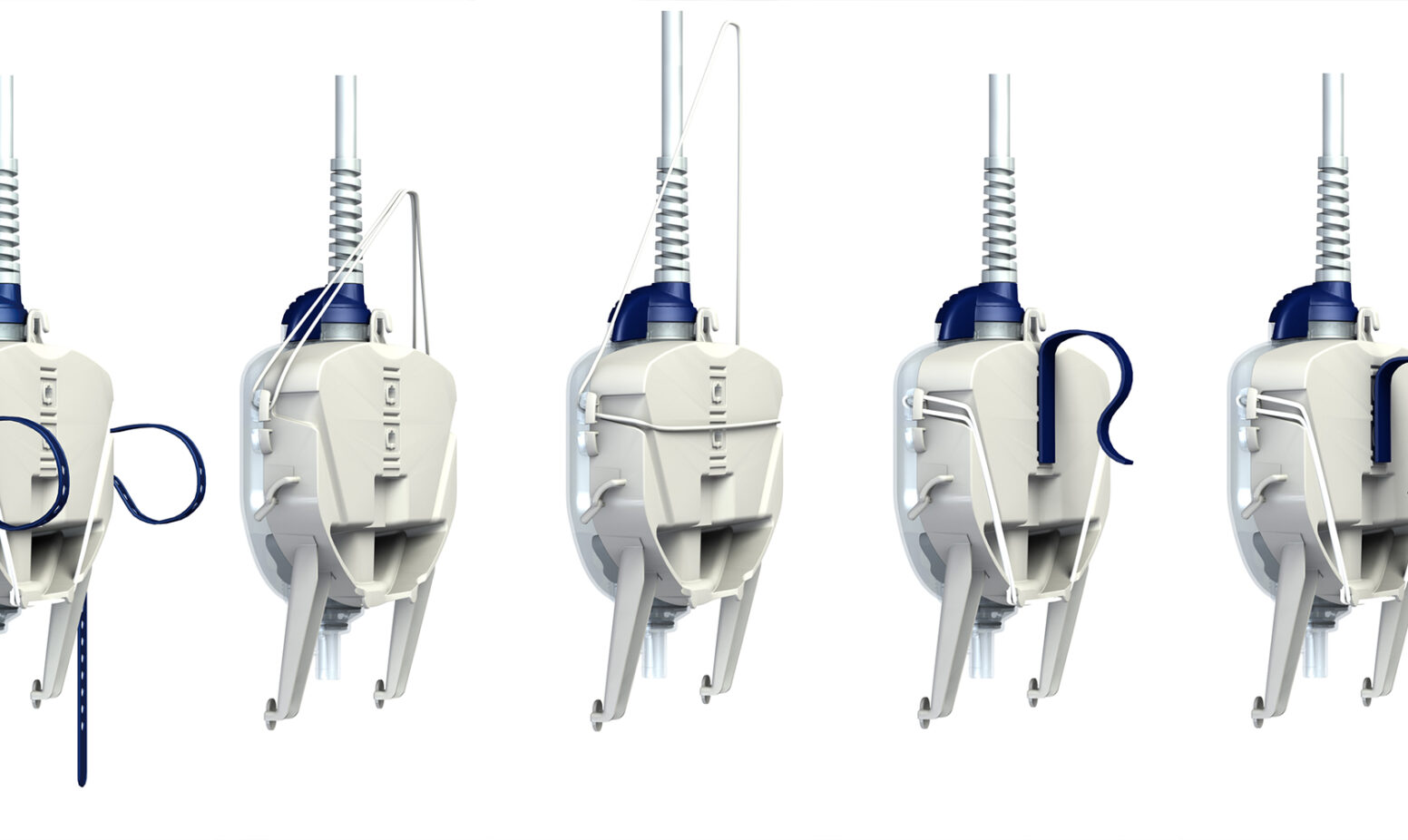
The PDD approach proves that successful product development can be evolutionary rather than revolutionary, so long as the designers understand and satisfy the needs of users.
The UnoMeter™ Safeti™ Plus won a Gold DBA Design Effectiveness award for its user centric design and commercial success.
The PDD design:
- Improved speed and versatility of fitting
- Optimised flow to reduce time to operate
- Made appearance more friendly and contemporary
- Added no cost to build
- On launch sales were +50%
- Project payback was 3 months.
- It became the top Unomedical vitality index product.
Since its launch, the UnoMeter™ Safeti™ Plus had an immediate effect... [and was] the single biggest driver of the company’s new product sales.